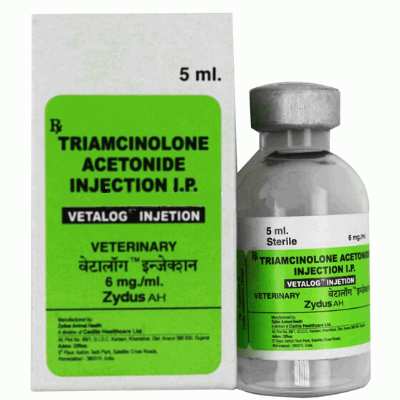
Vetalog Injection
Vetalog injection comes with Triamcinolone acetonide as an active ingredient. It is an intermediate-acting glucocorticoid and 4 – 10 times more potent than hydrocortisone. Intramuscular injection duration of activity may persist up to 6 weeks. It is indicated in Bovine Ketosis, Arthritis, Tenosynovitis, and related disorders, Dermatological Disorders including pruritus, Allergic reactions and dermatoses, Haematoma, Inflammatory conditions, and Pregnancy toxaemia in ewes/sheep.
Description
Vetalog Injection
Brand Name: Vetalog (Injection)
Manufactured by: Zydus AHL
Dosage form: Injection
Presentation: Vial of 5 ml
Ingredients & Composition:
Each 1 ml of Vetalog injection contains:
Triamcinolone Acetonide IP 6 mg
Benzyl Alcohol IP 0.9% v/v as a preservative
Water for injection IP Q.S.
Description & Mechanism of Action:
Vetalog injection comes with Triamcinolone acetonide as an active ingredient. It is an intermediate-acting glucocorticoid and 4 – 10 times more potent than hydrocortisone. Intramuscular injection duration of activity may persist up to 6 weeks.
Class: Steroid / Glucocorticoid
Indication / Uses:
- Bovine Ketosis
- Arthritis
- Tenosynovitis and related disorders
- Dermatological Disorders including pruritus
- Allergic reactions and dermatoses
- Haematoma
- Inflammatory conditions
- Pregnancy toxaemia in ewes/sheep
Doses and administration:
Dogs and cats: 0.1 – 0.2 mg /kg Body weight
Cattle and Horses: 12 – 20 mg (Total dose)
The effect generally persists for 7 – 15 days. If symptoms reappear, can be repeated.
Route of Administration: Intramuscular, Subcutaneous or Intra-articular route
Overdoses / Side effects / Contraindications /Warnings:
Use in the appropriate dosage. Primary adverse effects are cushingoid in nature with prolonged use. Use of triamcinolone is contraindicated in systemic fungal infections, animals with arrested tuberculosis, peptic ulcer, acute psychoses, corneal ulcer, & Cushingoid syndrome. The presence of diabetes, osteoporosis, chronic psychotic reactions, predisposition to thrombophlebitis, hypertension, CHF, renal insufficiency, & active tuberculosis necessitates carefully controlled use. In dogs, polydipsia (PD), polyphagia (PP), and polyuria (PU) may all be seen with short-term “burst” therapy as well as with alternate-day maintenance therapy on days when giving the drug.
Adverse effects in dogs like dullness, dry hair coat, weight gain, panting, vomiting, diarrhoea, elevated liver enzymes, pancreatitis, GI ulceration, lipidemias, activation or worsening of diabetes mellitus, muscle wasting, and behavioural changes (depression, lethargy, viciousness) can be observed. (reference: Plumb’ Therapeutics 6th edition).
Do not self medicate to avoid side effects and complications
Pharmaceutical precautions / Instructions:
Store in a cool and dry place at 15-30 °C. Protect from light. Shake well before use.
Safety: Age / Pregnancy/ Withdrawal:
Over and excessive dosages early in pregnancy may lead to teratogenic effects. In horses and ruminants, exogenous steroid administration may induce parturition when administered in the latter stages of pregnancy. It has potential withdrawal side effects if withdrawn without phasing or appropriate gradual reduction.
Habit-forming:
May need to be given for a longer period of time and may need a gradual reduction in dosage to avoid potential side effects and Cushingoid syndrome-like symptoms.
Substitute: Vetalog Parenteral (Boehringer Ingelheim)
Also see Dexona Injection, Prednisolone Injection, Cortalife Isoflupredone Injection, Isoflud Injection
Only logged in customers who have purchased this product may leave a review.





Reviews
There are no reviews yet.