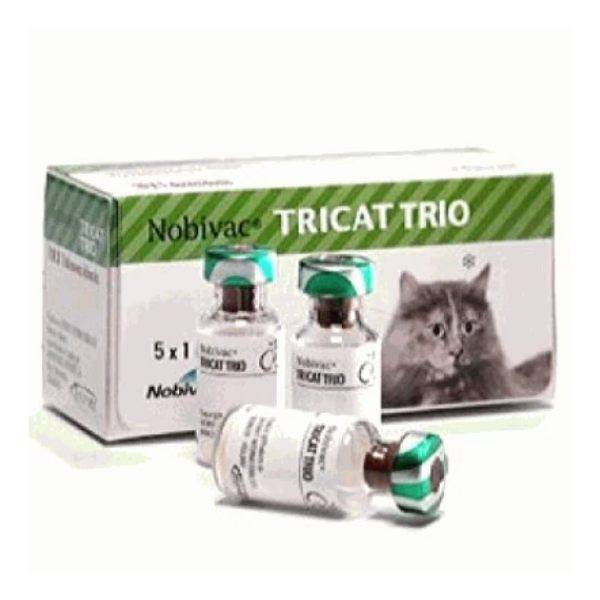
Nobivac Tricat Trio
Nobivac Tricat Trio is a live attenuated, freeze-dried vaccine containing the 3 core viruses – feline panleucopaenia virus (MW-1), feline herpesvirus type 1 (rhinotracheitis), and feline calicivirus. Clinical signs and virus excretion caused by infection with feline calicivirus and/or feline rhinotracheitis virus can be reduced and prevented including clinical signs, virus excretion, and leucopenia caused by feline panleukopenia virus with active immunization of cats with Nobivac Tricat Trio.
Description
Nobivac Tricat Trio
Brand Name: Nobivac Tricat Trio
Manufactured by: MSD Animal Health (Intervet)
Dosage form: Injectable (Freeze-dried Plug with Diluent)
Presentation:
1 ml (1 Dose) x 5 Vials – Box
In combination with an equal number of vials with Nobivac Solvents.
Nobivac Tricat Trio and its solvent come in a polyethylene terephthalate (PET) tray pack.
Ingredients & Composition:
Per dose of 1 ml reconstituted Nobivac Tricat Trio vaccine contains live attenuated:
Feline calicivirus, strain F9 at least 4.6 log10 PFU*
Feline rhinotracheitis virus, strain G2620A at least 5.2 log10 PFU*
Feline panleucopenia virus, strain MW-1 at least 4.3 log10 TCID50**
* PFU – Plaque forming units
** TCID – Tissue culture infectious dose
Description / Action:
Nobivac Tricat Trio is a live attenuated, freeze-dried vaccine containing the 3 core viruses – feline panleucopaenia virus (MW-1), feline herpesvirus type 1 (rhinotracheitis), and feline calicivirus. This is a combination vaccine with live attenuated strains grown in cell culture and presented as a freeze-dried plug. Each vial contains a single dose for reconstitution with solvent.
Nobivac Tricat Trio vaccine is exclusively for feline patients. Clinical signs and virus excretion caused by infection with feline calicivirus and/or feline rhinotracheitis virus can be reduced and prevented including clinical signs, virus excretion, and leucopenia caused by feline panleukopenia virus with active immunization of cats with Nobivac Tricat Trio.
Class: Biologicals / Feline Vaccine
Indication / Uses:
Nobivac Tricat Trio is indicated for active immunization of healthy cats against
- Feline Panleucopaenia Virus (FPLV),
- Feline Herpes Virus (FHV) And
- Feline Calicivirus (FCV).
- Vaccination will reduce clinical signs caused by infection with feline rhinotracheitis virus / Feline herpesvirus (FHV) and feline calicivirus (FCV); reduce replication of FCV, and prevent shedding of feline panleukopenia virus (FPLV).
Dosage and administration:
The contents of one vial of reconstituted vaccine i.e. 1 mℓ of reconstituted vaccine should be injected subcutaneously. Reconstitute immediately prior to use by addition of one vial of Nobivac Tricat Trio Diluent.
Contamination of vaccine with traces of chemical sterilizing agents must be avoided. Disinfectant or spirit should not be used to disinfect the skin prior to inoculation.
Vaccination Programme:
Core feline vaccination
Every healthy cat should be vaccinated against 3 core feline diseases – feline panleucopaenia (or infectious enteritis), feline herpesvirus (rhinotrachitis) and feline calicivirus (both causes of “cat flu”). The devastating consequences of these diseases for a cat can easily be prevented by regular vaccination.
The cat flu viruses are very contagious and widespread. Vaccination against cat flu can be tailored to the individual’s needs. This vaccine programme offers the flexibility of 3 yearly revaccination intervals using Nobivac Tricat Trio. It can be combined with targeted annual revaccination with Nobivac Ducat in interim years for those cats at higher risk of exposure to cat flu.
- For a cat at higher risk of exposure – one that visits boarding catteries, has an indoor-outdoor lifestyle or is from a multi-cat household – annual vaccination is recommended.
- For a cat at lower risk of exposure – an indoor cat that doesn’t visit boarding catteries – 3 yearly vaccination is adequate.
For an initial vaccination: Two doses are required, at an interval of 3 to 4 weeks.
The first dose of vaccine is given from the age of 8-9 weeks and the second inoculation from the age of 12 weeks.
In the case of earlier vaccination where it is desirable or necessary, vaccination is appropriate from 6 weeks of age onwards but should be followed by the normal vaccination scheme at 8-9 and 12 weeks of age.
Only healthy cats should be vaccinated, and an adequate clinical examination should be made prior to inoculation.
Duration of immunity: The duration of immunity is 3 years for the FCV, FHV, and FPLV components, as confirmed by the challenge study.
While the duration of immunity is 1 year for the FCV and FVR components; 3 years for the FPLV component.
Overdoses / Side effects / Contraindications /Warnings:
Slight temporary swelling at the site of injection can be observed for one day. A slight temporary rise in rectal temperature (< 40 °C) may occur, while occasionally slight discomfort may be observed during the first day after vaccination.
Strictly avoid mixing of Nobivac Tricat Trio with other vaccines.
Accidental self-injection may lead to a severe local allergic reaction. Consult a physician and show this package insert.
Like other vaccines, anaphylactic or hypersensitivity reactions may occur. Although it has been extensively tested under a large variety of conditions failure thereof may ensue as a result of a wide range of reasons. If vaccination failure is suspected, seek veterinary advice, and notify the registration holder. Observe aseptic precautions.
Pharmaceutical precautions / Instructions:
The vaccine must be stored in the original container between 2 °C and 8 °C. Protect from light. Solvent Can be stored at 15-25°C if stored independently from the vaccine. Care should be taken to avoid repetitive exposure to high ambient temperatures or prolonged exposure to high temperatures following withdrawal from the refrigerator prior to use. Vaccine potency in hot summer conditions can be severely reduced within a few hours. Do not freeze. Use sterile injection equipment. Shake before and during use. For animal treatment only. No Special precautions to be taken by the person administering the veterinary medicinal product to animals.
In the case of human exposure, contact a physician.
Waste material should be disposed of by boiling, incineration, or immersion in an appropriate disinfectant approved for use by the competent authorities. Do not store partially used containers for future use. Use the entire contents when opened and dispose of any unused vaccine as well as all empty vaccine containers and vaccination equipment according to local waste disposal regulations. Keep out of reach of children, uninformed persons and animals.
Dissolve the freeze-dried plugin Nobivac Tricat Trio Solvent. Allow to reach room temperature before use. Ensure complete dissolution of the freeze-dried plugin Nobivac Tricat Trio Solvent prior to use of the vaccine. Avoid intravenous injection. It is good vaccination practice when handling vaccines to avoid contact with the eyes, hands and clothing.
Safety: Age / Pregnancy/ Withdrawal:
Do not use in kittens less than 6 weeks of the age. The recommended age for the first inoculation is 8 – 9 weeks. Use during pregnancy and lactation: As this product has not been tested in pregnant queens and during lactation, vaccination during pregnancy and lactation is not recommended. • Vaccinate healthy cats only.
Withdrawal period is not applicable.
Always follow the recommended dose.
Habit forming: No, But Protocol should be followed according to the guidelines.
Substitute: Feligen CRP, Felocell
Only logged in customers who have purchased this product may leave a review.



Reviews
There are no reviews yet.